
Water Analysis - Hypothetical Combinations
Suppose for a minute we analyze the total mineral content of a typical water sample which has nine grains of minerals per gallon. It could well be water collected from Chicago, Detroit, Cleveland or any of a number of other cities drawn from the Great Lakes.
| CATIONS | ANIONS |
| Ca 5.0 gpg* | HC03- 7.0 gpg* |
| Mg 2.5 gpg* | SO4- 1.0 gpg* |
| Na See note | Cl-1.0 gpg* |
| *as CaC03 |
Diagrammed, these minerals would appear as shown on the chart below:
Diagram of Mineral Concentration of Water with 9 Grains of Total Minerals
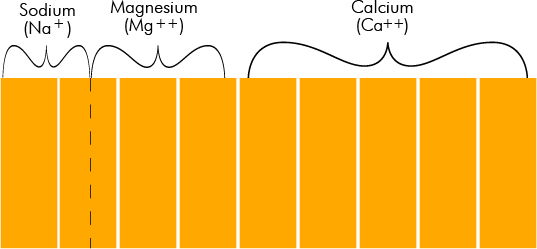
NOTE: Analysis for sodium is not usually made directly in a water analysis. Its concentration is estimated by the difference between the total of the anions and the total hardness.
EXPLANATIONS
The bar at the left in the graph represents the cations of positive ions of the various minerals in the solution.
The bar at the right represents the anions or negative ions.
Remember that in all compounds the sum of the positive charges equals the sum of the negative charges. As a water analysis report simply gives the total of various compounds, the same holds true.
Our sample shows positive ions as follows: 5.0 gpg calcium, 2.5 gpg magnesium, 1.5 gpg sodium for a total of 9.0 gpg. The compensating negative ions are: 7.0 gpg bicarbonate, 1.0 gpg sulfate and 1.0 gpg chloride.
A chemist making an analysis of this 9 grain water could report its dissolved minerals in the following manner.

