
- Allergies and Air Pollution's Effect on Health
- Asthma & How it is affected by Air Pollution
- Air Pollution & Cancer
- Air Pollution's Harmful Effects on the Elderly
- More about HEPA and Carbon activated Filters
- Different types of air filters
- Mold Spores and Indoor Air Quality
- UV Air Purifier Guide
- What causes bad indoor air quality?
- How do Air Purifiers Work?
- Air Pollution May Cause Respiratory Infections
- Air Pollution in the workplace
- Pet Dander's Effect on Air Quality and Asthma
- Air Pollution & Sleep Apnea
- Top 5 Reasons Why you should purify Air
- What you need to know about indoor air
- How Pets cause allergies
- Tips on Choosing the Right Air Purifier
- Multiple Chemical Sensitivity
- What are Dust Mites?
- The History of Air Purifiers
- Air Pollution Problems of the new home
- 5 Ways to Reduce Your Pollen Allergies
- Sinusitis Causes and treatments
- What is HEPA filtration?
- Air purifiers and Wood Smoke
- Home Air purifiers and Cigarette Smoke
- Volatile Organic Compounds (VOCs)
- Formaldehyde Air Pollution
- Is your air purifier ozone free?
TOXIC WATER: A Guide to Acid Rain |
 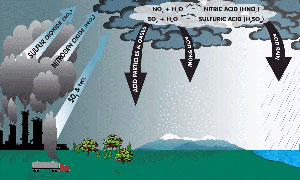 Acid Rain (Mouse over to see larger picture) |
Acid rain is defined as rain or another kind of precipitation that is abnormally acidic. This means that acid rain has heightened levels of something called hydrogen ions. Acid rain is known to have hurtful impacts on aquatic animals, plants and even infrastructure by a wet-disposition process. Emissions of nitrogen oxides and sulfur dioxide cause acid rain, and various governments have made an effort for decades to lessen sulfur dioxide’s release into the atmosphere. Chemicals in acid rain have been known to cause the paint to actually peel from surfaces.
History of Acid Rain in the US
Acid rain is a widely used phrase that really refers to the deposition of dry (acidifying gases and particles) and wet (snow, rain, fog, sleet, dew or cloud water) acidic components. Back in the 17th century, the English gardener by the name of John Evelyn first remarked the bad effects of city air that was acidic. His observations centered upon the Arundel marbles and their poor condition. In the U.S., the Congress first passed the Acid Deposition Act back in 1980, which set up a 10-year program of research directed by the National Acidic Precipitation Assessment Program. In 1990, the Congress passed some amendments to the Clean Air Act that included Title IV, which set up the Acid Rain Program, a system of cap and trade meant to regulate emissions of nitrogen oxides as well as sulfur dioxide. The goals of this Program have been successful according to the Pacific Research Institute, with the levels of acid rain falling by 65 percent.
Emissions of Chemicals
Sulfur dioxide is the most significant gas when it comes to acidification. Natural phenomena also can contribute to acid rain; the biggest contributor would be volcanoes. For instance, the fumaroles in the crater of Laguna Caliente of Poas Volcano produce very high quantities of fog and acid rain, which has the effect of ridding the surrounding area of any vegetation and even stinging the eyes and lungs of nearby residents. However, when it comes to the main source of acid rain, it actually can be traced back to human activity that produces sulfur and nitrogen compounds. For example, motor vehicles, factories and the generation of electricity are all producers of sulfur and nitrogen compounds. Even the production of livestock is considered a human activity that contributes to and creates acid rain.
Chemical Processes
The combustion of fuels generates both nitric oxides and sulfur dioxides, which are in turn converted into nitric acid and sulfuric acid. In its gas phase, sulfur dioxide becomes oxidized through a reaction with the hydroxyl radical; this occurs through an intermolecular reaction. In a case where clouds are present, the rate of loss of sulfur dioxide is quicker than can be accounted for by only gas phase chemistry. The reason for this relates to responses in the liquid water drops. There are many aqueous reactions which oxidize sulfur from one state to another, and this leads to the creation of sulfuric acid. The most significant reactions involving oxidation have to do with the ozone, oxygen and hydrogen peroxide.
Acid Deposition
Acid deposition occurs in both dry as well as wet deposition. Dry acid deposition happens in about 20 to 60 percent of all acid deposition cases. Dry acid deposition happens when gases and particles stick to the ground, surfaces or plants. Wet acid deposition happens when any type of precipitation takes away acids from the Earth’s atmosphere and brings it to the surface of the Earth. This can occur because of the precipitation taking away acids below clouds or within clouds or the acid deposition created in raindrops.
The Effects of Acid Rain
The effects of acid rain have been nothing but adversarial, with affects hurting soils, freshwater, forests, insects, aquatic life forms, buildings and the health of people. Fish and other aquatic animals are injured by acid rain because of all the aluminum concentrations found in surface water as a direct result of acid rain. Acid rain has even been lethal for some species like the brook trout, eliminating it altogether in some streams and lakes that are geographically sensitive areas. Even soil biology and soil chemistry can be poisoned by acid rain. When this occurs, some of the microbes’ enzymes in soil get denatured (meaning they alter their shape and so no longer function properly). When acid rain impacts soil chemistry, it can harm sensitive species like the sugar maple.
One of the most concerning effects of acid rain is its effect on humans, though, thankfully, acid rain does not affect humans straightaway. However, the particulates that are the cause of acid rain (nitrogen oxides and sulfur dioxide) do have a harmful effect on humans. The presence of the aforementioned particulates in the air can contribute to both lung and heart issues in people. Plants and forests can be hurt by acid rain, and acid rain also hurts structures such as historic monuments and regular buildings. Structures that are constructed out of marble or limestone are really vulnerable due to the amount of calcium carbonate in them.
Regions Affected
Because rainfall occurs everywhere, acid rain affects a good number of regions throughout the world. In fact, acid rain occurs all over the globe, but it occurs with more frequency and impact in some places as opposed to other places. For example, Europe has its fair share of acid rain, which includes Poland and goes all the way to Scandinavia. North America has its own share of acid rain ordeals, with the eastern third portion of the U.S. and the southeast of Canada all being affected by it. The continent of Asia is not spared the effects of acid rain, and prominent countries in Asia that suffer acid rain are Taiwan and China’s southeastern coast.
Prevention
Preventing acid rain comes down to three different approaches: technical solutions, emissions trading, and international treaties. Technical solutions include approaches such as flue gas sulfurization to take away sulfur-possessing gases from the stack gases. Flue gas desulfurization has been shown to take away up to 95 percent of the sulfur dioxide in the flue gases. International treaties include agreements like the Air Quality Agreement and the Sulphur Emissions Reduction Protocol. These agreements are an attempt to reduce the long-range emission of atmospheric pollutants. Finally, regulatory schemes such as emissions trading are another way that countries have attempted to prevent acid rains. Regulatory schemes propose that factories buy an emissions allowance for every unit of a pollutant that they emit, and they can then sell off parts of their allowance if they do not use it all up.

