Among all common water filtration methods, the process used for the removal of all dissolved salts from water is referred to as deionization. Deionization requires the flow of water through two ion exchange materials in order to effect the removal of all salt content.
The terms demineralization and deionization are used somewhat interchangeably by the industry. While the term demineralization is generally better understood, deionization is especially apt.
The passage of water through the first exchange material removes the calcium and magnesium ions just as in the normal softening process. Unlike home equipment, deionization units also remove all other positive metallic ions in the process and replace them with hydrogen ions instead of sodium ions.
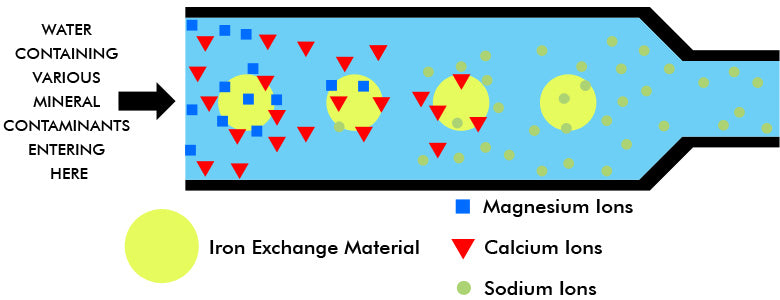
As the metallic ions in the water affix themselves to the exchange material, the latter releases its hydrogen ions on a chemically equivalent basis. A sodium ion (Na+) displaces one hydrogen ion (H+) from the exchanger; a calcium ion (Ca++) displaces two hydrogen ions; a ferric ion (Fe+++) displaces three hydrogen ions, etc. (Recall those home softeners also release two sodium ions for every calcium or magnesium ion they attract.)

This exchange of the hydrogen ions for metallic ions on an equivalent basis is a chemical necessity that permits the exchange material to maintain a balance of electrical charges.
Now because of the relatively high concentration of hydrogen ions, the solution is very acidic.
At this point the deionization process is just half complete. While the positive metallic ions have been removed, the water now contains positive hydrogen ions, and the anions originally in the raw water.
The partially treated water now flows through a second unit, this time an anion exchange material normally consists of replaceable hydroxyl anions and fixed irreplaceable cations.
Now the negative ions in the solution (the anions) are absorbed into the anion exchange material. Released in their place are hydroxyl anions.
All that emerges from such a two-unit system is ion-free water. It still contains the positive hydrogen ions released in the initial exchange plus the negative hydroxyl ions released in the second exchange.
What has become of these two ions? Through the magic of chemistry, they have combined (positive to negative) to produce water molecules that are in no way different from the water in which they were produced.
The result of this two-stage ion exchange process is water that is mineral-free.
Equipment for use in the deionization process may be of several types. Available are both multiple beds and single bed units. Multiple bed units have pairs of tanks, one for the cation exchanger, the other for the anion exchanger. Single bed units incorporate both the cation and anion exchangers, mixed in a single tank.
Deionized water has a wide range of uses in industry. Chemical production, pharmaceuticals, electroplating, television tube production, and leather goods processing are among the many diversified applications for deionized water.
Efforts to produce mineral-free water through multiple distillations have proved to be extremely complex and require elaborate and costly equipment.



